A Complete Guide to Medical Device Leak Testing
Medical Devices
A medical device can be defined as a tool or apparatus designed to be used for medical purposes. These devices aid in the diagnosis, prevention, monitoring, and treatment of diseases as well as perform functions that support the sustenance of life. According to the world health organization, there are over 2 million different kinds of medical devices. These devices vary in size, shape, materials, and complexity of their function and components.
In the medical device manufacturing market, devices typically have a barrier and pathway-like function that requires keeping whatever is inside the device (such as a gas or liquid for example) inside while simultaneously preventing whatever is outside the device from getting inside it. These devices are commonly used to transport or extract fluids or gases to and from specific parts of the body. Due to the important function and nature of these devices; it is imperative that they undergo stringent and accurate leak testing to ensure each medical device functions as it should, is safe to use, and meets the highest standards of quality.
ATEQ offers instruments to address a large range of test methods and requirements. Our products can be used in manual benchmark and automated environments. They are also fully customizable to meet the specific testing parameters of thousands of parts.


Why Detecting Leaks in Medical Devices Matters
Using our previous example, if a leak existed in a medical device this could result in fluids or gases seeping or leaking out into the wrong parts of the body. This can result in severe consequences for the end users (patients and healthcare professionals) and can even be fatal! In addition, the manufacturer could also be held responsible for the failed medical component resulting in lawsuits, fines, and recalls which could cost the company millions of dollars. Medical devices produced by manufacturers must meet ever-expanding quality and safety parameters to comply with the strict regulations and standards of the FDA and GMP. The best way for manufacturers to mitigate risk is by identifying components that don’t meet production standards using leak testing instruments that are accurate, scalable, and repeatable. Therefore, leak testing has become a critical part of the assembly and production line process.
Methods of Leak Testing for Medical Devices
1. The Air Bubble Method
The air bubble method is a traditional method of leak testing that uses water. A medical device or part is first filled with air or pressurized internally. It is then submerged under water if a leak is present, bubbles will then form and rise to the surface. This method is straightforward yet very slow as an operator must individually submerge each part in the water. Another factor that makes this method time-consuming is the clean-up because each part must be submerged under water each part must also be cleaned and dried sufficiently after testing is completed. Manufacturers typically produce hundreds of thousands of medical devices and parts daily, making this method inefficient for most plants today.
2. Pressure Decay Method:
The pressure decay method is one of the most used methods to leak-test medical devices. Pressure decay methods measure the difference in positive pressure or negative air pressure. First, we will explore the positive pressure decay method; It works by pressurizing (or filling) the medical device or “test part” with a specified amount of dry, clean, air. After a short stabilization period, a pressure decay instrument would then continue to monitor if the air pressure decreases over a set amount of time. If the pressure measured within the test part drops during the test this would indicate the presence of a leak.

3. Vacuum Decay Method:
Vacuum decay operates essentially in the opposite manner to the positive pressure decay method. As mentioned above, in a positive pressure decay leak test the test part is filled with air and a decrease in pressure indicates that a leak is present. This is considered positive pressure because you are increasing the air within the part while monitoring if there is a decrease in air pressure. Whereas in a vacuum decay leak test, you place a device or component within a test chamber and vacuum or extract the air surrounding the part. If the instrument performing the leak test indicates that the pressure within the chamber has increased that would indicate that the test part leaks. Just like in the case with the method above if the part has an allowable acceptable leak rate, then the difference between the initial vacuum and final vacuum would be used in determining if the part will pass or fail the test.

4. Flow Test Method:
The flow test method measures the rate at which air is flowing or passing through a part. When performing a flow leak test the test part or component is pressurized and then clean, dry air is pushed through the part. The flow test instrument measures the rate at which air is flowing through the part and how much air is needed to maintain the pressure inside of the part. The more air that is needed to maintain the internal pressure the bigger the leak. The flow test method detects leaks but also detects occlusions or blockages within the parts. This method of testing is very important for medical devices such as catheters, ventilator tubes, and other critical flow requirements. Our D620 flow tester has the latest leak testing technology that will guarantee you have the fastest and most accurate leak testing cycles in the market.

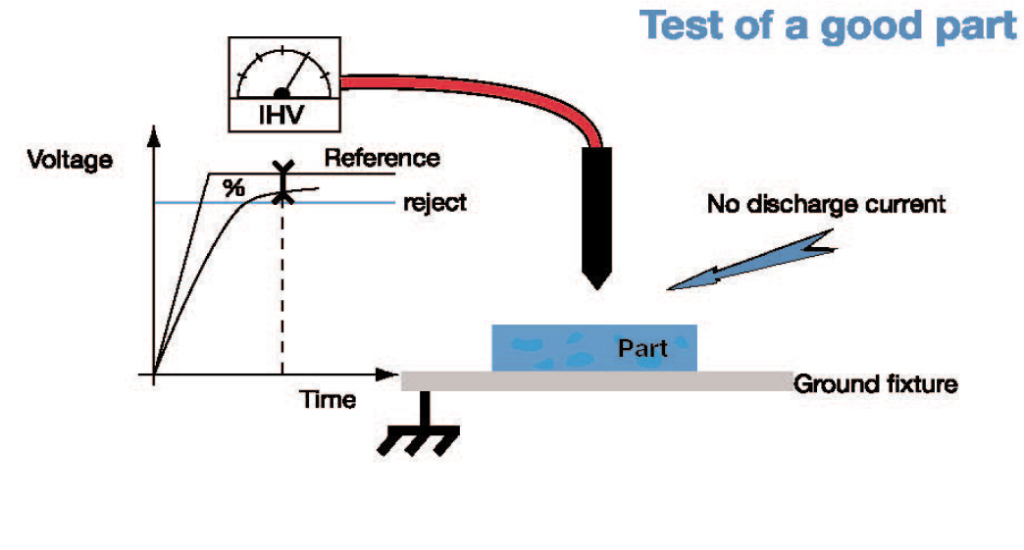
5. IONIQ Electrical Method:
The Ioniq leak testing method measures the current of ions flowing between a charged electrode and a grounding plate that is placed under the part that is being tested. If there is a leak detected the charged ions will flow through the leak or hole to the grounding plate. If there is no hole or weakness detected the measured voltage and nominal voltage will be virtually equal at a high percentage for instance 100%. However, if there is a hole and the test part is below the reject level the measured voltage will be measured as significantly lower than the nominal voltage, and will show a low percentage for instance 20%. ATEQ’s Ioniq has been developed to use this technology for the high production volume of plastic parts. Medical applications include but are not limited to the testing of plastic bottles, caps, batteries, and condoms as this instrument is used to detect membrane thickness, perforations…etc.
Leak and Flow Testing Solutions for Medical Device Manufacturing
From its position as the world leader in Leak Test technologies for assembly lines, ATEQ has developed a whole range of industrial quality control solutions to help you at every stage of your medical device assembly. We pride ourselves on only providing the best instruments, solutions, service, and expertise to our more than 5000+ customers around the world. If you have questions about the appropriate leak and flow testing methods for your medical manufacturing needs contact us to talk to an engineering specialist.